Sexually transmitted infections
Gonorrhoea is among the three most common sexually transmitted infections, and its resistance to antibiotics is rapidly spreading. There is now only one remaining last-resort treatment for gonorrhoea. If we continue down the current path, this treatable disease may become untreatable. GARDP is acting now to develop a new antibiotic for gonorrhoea infection in patients with limited treatment options.

Programme goals

A new, accessible treatment for gonorrhoea, a disease that affects 82 million people globally each year.

Explore different interventions for STIs, in line with GARDP’s 2024-2028 Strategy.
“To keep pace with the inevitable emergence of drug resistance to the few treatments currently available, we must invest now in developing new antibiotics, researching further existing antibiotic regimens, and accelerating equitable antibiotic access.”
– Alison Luckey, Associate Director Medical Sciences

Current project
Zoliflodacin drug development project
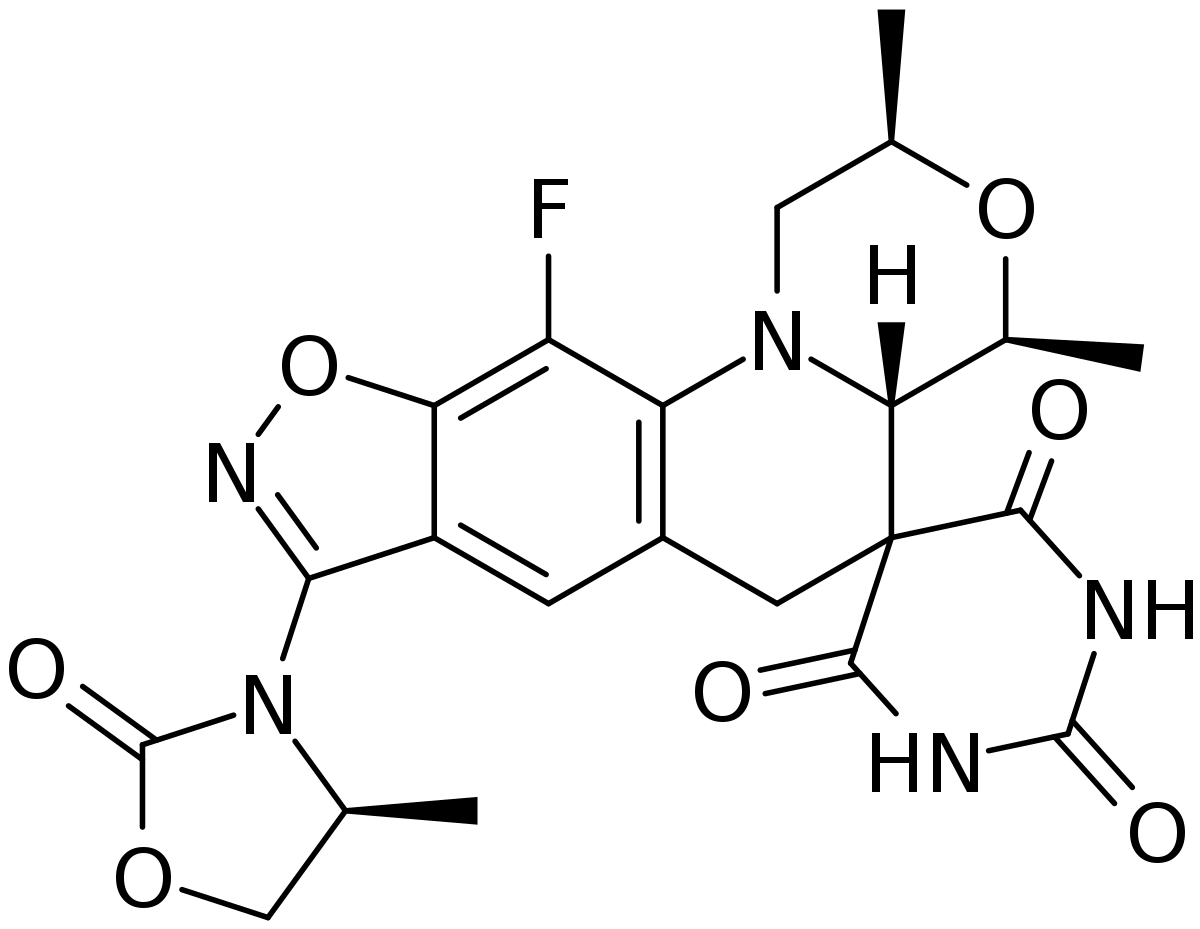
In December 2025 the US Food and Drug Administration (FDA) approved Nuzolvence® (zoliflodacin) for oral suspension, a new first-in-class, oral, single-dose antibiotic for the treatment of uncomplicated urogenital gonorrhoea. Co-developed by GARDP in partnership with Innoviva Specialty Therapeutics, zoliflodacin is the first new treatment in decades to be developed solely for gonorrhoea, and the first antibiotic to be developed using a novel not-for-profit approach to antibiotic R&D aimed at tackling the rise and spread of AMR.
Neisseria gonorrhoeae has developed resistance to almost all antibiotics used to treat it, with only one last remaining recommended antibiotic treatment, ceftriaxone. Now we are seeing resistance even to this, with a six-fold increase of these kinds of resistant infections in some regions in recent years. Without new and effective antibiotics, like zoliflodacin, gonorrhoea is in danger of becoming one of the first diseases to become once again untreatable because of AMR.
Zoliflodacin belongs to a new class of antibiotics, called spiropyrimidinetriones, which has a unique mechanism of action in the way that it inhibits a crucial bacterial enzyme called type II topoisomerase, which is essential for bacterial function and reproduction. In previous in vitro studies, zoliflodacin has been shown to be active against all multidrug-resistant strains of N. gonorrhoeae tested, with no cross-resistance with other antibiotics.
Zoliflodacin is being developed exclusively for the treatment of gonorrhoea. By focusing solely on this indication, the aim is to limit the clinical use of this new treatment to the targeted infection only. This approach can help to minimize the likelihood of excessive use, which could potentially contribute to the development of resistance, and therefore preserve the usefulness of zoliflodacin in treating gonorrhoea infections for longer.
The approval of zoliflodacin follows a pivotal phase 3 clinical trial, that was sponsored and led by GARDP, and met its primary objective when compared against the current global standard of care. The findings of this trial were published in The Lancet in December 2025. Involving 930 participants across 16 trial sites within five countries in four continents – including Belgium, the Netherlands, South Africa, Thailand, and the U.S. – the trial is the largest ever conducted for a new treatment against gonorrhoea, it was also one of the most geographically and demographically diverse. It was also designed to be conducted in regions with high prevalence of gonorrhoea and include underrepresented populations, such as women, adolescents and people living with HIV.
GARDP has the right to register and commercialize zoliflodacin in more than three-quarters of the world’s countries, including all low-income countries, most middle-income countries, and several high-income countries. In November 2025, zoliflodacin was submitted for priority review in Thailand, with a submission in South Africa planned for early 2026. GARDP is committed to generating more data where required to support expanded access to zoliflodacin.



Key milestones
- SIGNED collaboration agreement with Entasis to develop and register zoliflodacin, with the goal to provide access to patients who suffer from drug-resistant gonorrhoea in low- and middle-income countries
- ENROLLING patients at all sites in phase 3 trial of zoliflodacin
- COMPLETED manufacturing of the three batches of zoliflodacin for new drug registration
- SIGNED Memorandum of understanding with Dr Reddy’s Laboratories and Aurigene Pharmaceutical Services Limited (APSL) in India, as well as with the Thailand Ministry of Public Health, to work toward improving access to zoliflodacin upon approval
- COMPLETED prevalence study of sexually transmitted infections, including gonorrhoea, in Kenya (results forthcoming)
- COMPLETED recruitment for global phase 3 trial of zoliflodacin
- COMPLETED pivotal phase 3 trial
- ANNOUNCED topline data for phase 3 trial
- ANNOUNCED US FDA acceptance of New Drug Application for zoliflodacin
- ANNOUNCED NUZOLVENCE® (Zoliflodacin) receives U.S. FDA Approval





About gonorrhoea
About gonorrhoea
Gonorrhoea is a widespread sexually transmitted infection that is treatable in most cases with antibiotics. However, resistant strains of Neisseria gonorrhoeae, the bacteria responsible for gonorrhoea, are on the rise all around the world, including in early 2022 three new cases of “super-gonorrhoea” in the UK alone. The World Health Organization has thus labelled Neisseria gonorrhoeae a priority pathogen in urgent need of new treatments.
If left untreated or when untreatable, gonorrhoea can cause serious, lifelong consequences in men and women, and can amplify the spread of HIV in high-prevalence settings.
The disease disproportionately harms women, who often fail to show early symptoms and thus do not seek treatment. When left untreated in women, gonorrhoea can lead to pelvic inflammatory disease that elevates the risk of complications in pregnancy, including the likelihood of ectopic pregnancies and infertility. During birth, gonorrhoea can be transmitted to babies, who in turn may have health problems like gonococcal conjunctivitis and skin infections.