Who we are
The Global Antibiotic Research & Development Partnership (GARDP) accelerates the development and access of treatments for drug-resistant bacterial infections. Together with public, private and non-profit partners, GARDP works to preserve the power of antibiotics for generations to come.

GARDP is acting now to counter antibiotic resistance and save lives
Antimicrobial resistance (including antibiotic resistance) is one of the top 10 global health threats. In 2021, 1.27 million people died as a result of drug-resistant bacterial infections, nearly as many as HIV and malaria combined. If left unchecked, antibiotic resistance may kill as many as 10 million people each year from 2050.





Centering antibiotic drug development on public health needs

We integrate as early as possible the constraints of public health needs and access challenges into the selection of our projects, so that these projects eventually benefit patients globally. We are directly involved in pharmaceutical and clinical development to ensure that every treatment we develop is safe, effective, affordable and suitable for use in diverse settings, including those with high AMR burden and limited resources.

We de-risk antibiotic drug development projects by negotiating collaboration and licensing agreements with pharmaceutical companies. In exchange for our expertise and financial support, we seek the rights to manufacture and distribute treatments, through sublicensees, in regions with high morbidity and mortality due antibiotic resistance.

Many low- and middle-income countries have expertise that is critical to address the global antibiotic crisis. Our public-private partnership model involves working with these individuals and all key stakeholders from the get-go to coordinate efforts in the antibiotic pipeline of drug development and access.
GARDP envisions a world where all infections are treatable for everyone, everywhere.

Impact to date
GARDP signed a first-of-its-kind license and technology transfer agreement with Shionogi and a collaboration agreement with Shionogi and CHAI that together aim to significantly transform the landscape of access to antibiotics for countries around the world.

GARDP is developing a portfolio of potential treatments for adults and children, including working with Bugworks Research Inc. on the clinical and pharmaceutical development of an investigational compound with activity against a broad spectrum of pathogens.
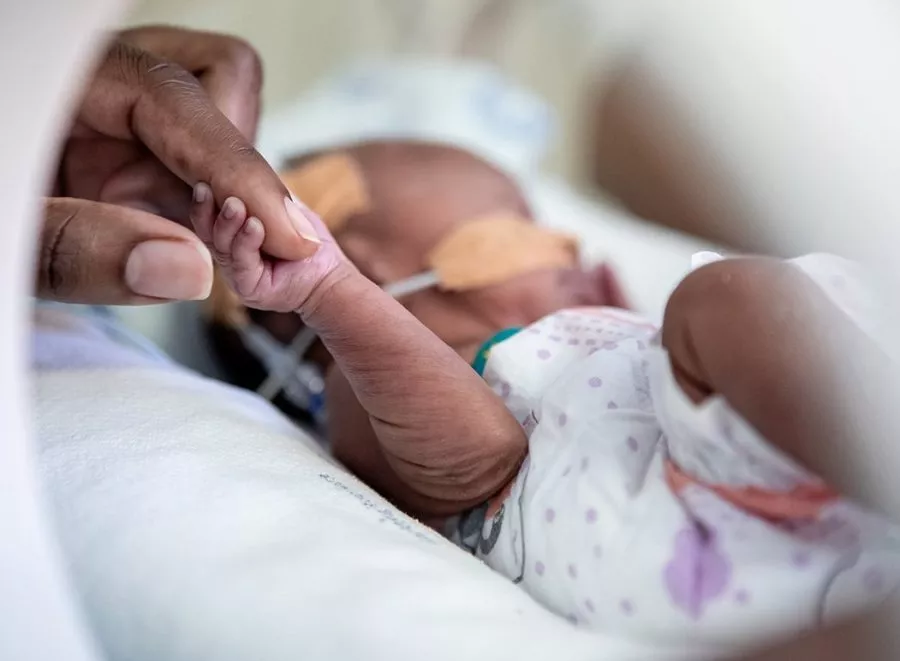
GARDP with partners completed one of the largest ever observational studies on the care of babies with sepsis—3,200 newborns in 11 countries worldwide. GARDP has used data from the study to design and launch “NeoSep1”, a trial to identify improved treatment regimens for newborns with sepsis.



GARDP has successfully completed a global phase 3 trial of a new first-in-class antibiotic, zoliflodacin, to treat uncomplicated gonorrhoea. If approved by the US FDA, it will be the first new antibiotic for treating gonorrhoea in decades.

GARDP has screened over 130,000 compounds plus extracts of natural compounds for antibiotic activity against two WHO priority pathogens. GARDP is now testing and optimizing chemical series (each corresponding to a promising compound) for possible development.

GARDP’s REVIVE is leading efforts to preserve and share scientific knowledge and tools for antimicrobial R&D at the global level, notably with freely available webinars that feature experts in the field from around the world.


Forging partnerships
Forging partnerships
GARDP partners with research and healthcare institutions around the world including those in regions that are heavily affected by drug resistance, drawing on local expertise and skills and building local capacity as needed.



“The rise of drug-resistant bacteria is jeopardizing decades of progress and threatening our ability to prevent and treat infections that were once easy to treat. The Global Antibiotic Research and Development Partnership is an essential element of delivering the Global Action Plan on Antimicrobial Resistance.”


An organization built for the 21st century

Committed to openness, transparency, honesty and accountability.

