Access to Antibiotics
The rise and spread of antimicrobial resistance (AMR) is often attributed to the historic overuse and inappropriate use of antibiotics. But their underuse also plays a significant role, with more people now dying from a lack of access to antibiotics than from drug-resistant infections. That is why achieving sustainable access to effective antibiotics is a priority for GARDP and central to everything it does. Because not only will it help to address the escalating global AMR crisis, but it will also save lives in the process, preventing more than 50 million deaths by 2050.
The lack of access to effective antibiotics is a global problem that acutely affects people across entire countries and regions, as well as certain population groups. A recent study found that the majority of the 18 new antibacterials approved and launched between 2010-2020 were accessible in only 3 out of 14 high-income countries (Sweden, UK and US). In low- and middle-income countries, the problem is even worse. Only 12 of the 25 new antibiotics that entered the market between 1999 and 2014 were registered in more than ten countries. Moreover, the evaluation of new treatments for use in children is regularly delayed for years, if undertaken at all.
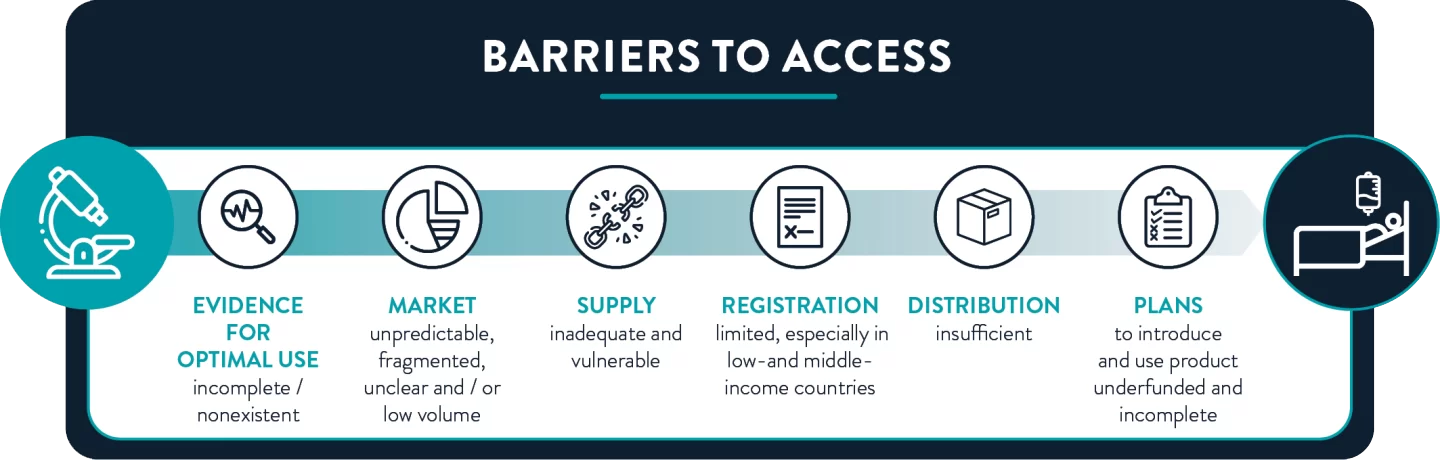
Impediments to antibiotic access range from lack of high-quality evidence for usage guidelines to incomplete introduction plans. Efforts to overcome these barriers—including access objectives in National Action Plans on antibiotic resistance—remain underfunded and largely unimplemented.
GARDP’s approach to access
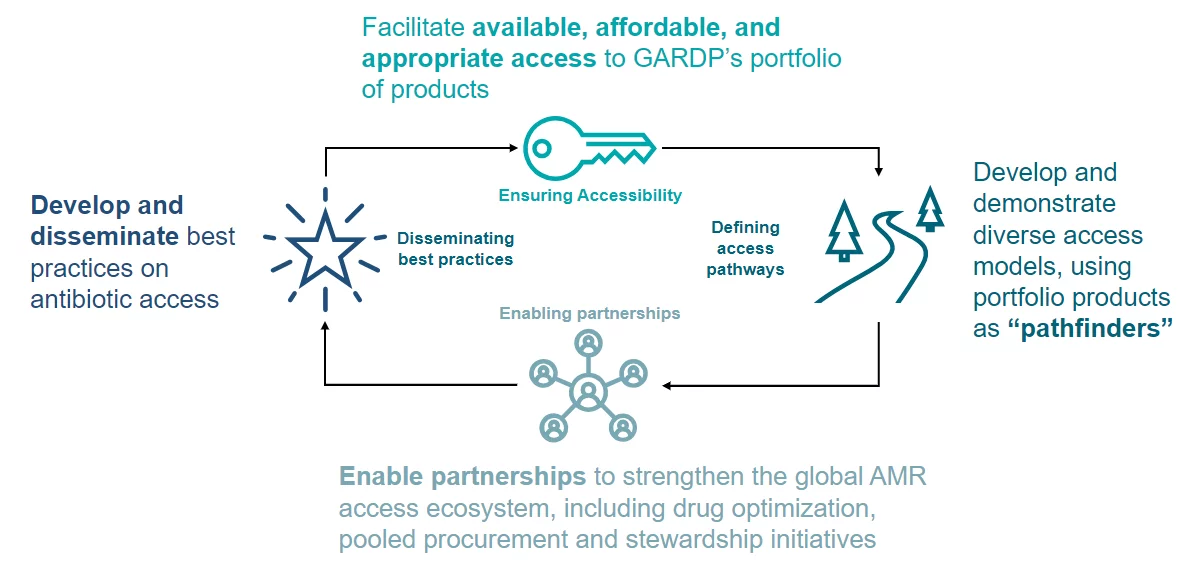
GARDP’s approach to access
For antibiotics to treat bacterial infections, patients and clinicians must first have access to them. GARDP is working to overcome barriers to access so patients of all ages can receive appropriate treatments wherever they live. We do this by working with local partners to carry out studies that will help fill vital data gaps to identify the antibiotic needs and inform introduction and appropriate use strategies for key high-burden countries.
At the same time, our work involves using licencing agreements with manufacturers and commercial partners, and working with regulatory authorities to accelerate the registration of antibiotics to support the supply of quality-assured and affordable antibiotics. We also ensure that access is factored into every aspect of our R&D efforts to develop effective new antibiotics. All this will ultimately increase access by improving the availability and in-country delivery of much-need antibiotics that target multidrug-resistant infections.
Programme goals

Improve access to new and existing antibiotics in GARDP’s portfolio that are effective against drug-resistant infections, by removing the barriers that prevent countries from introducing them.

Use GARDP’s portfolio products as pathfinders to create a new antibiotic research and development (R&D), and access ecosystem that factors access into every stage of the process, from scientific discovery and R&D, to the manufacturing, registration and introduction of treatments.

Support country efforts to address drug-resistant infections by expanding access to optimized antibiotic portfolios in a sustainable, equitable and appropriate way.
“The products that are coming out of the antibiotic development pipeline are not making it to patients around the world. This is especially true of patients in low- and middle-income countries, who are most affected by antibiotic resistance. Improving access to the antibiotics has to be the number one priority.”
– Jennifer Cohn, Global Access Director, GARDP

Current projects
Cefiderocol access project
Cefiderocol is a GARDP pathfinder project for access. Here GARDP is working with partners to expand access to cefiderocol, an antibiotic that is active against a number of drug-resistant bacteria on the World Health Organization (WHO) priority pathogen list. Normally, when antibiotics like cefiderocol are first developed, it can take more than a decade before people in low- and middle-income countries (LMICs) get access to them, if at all. This innovative partnership aims to change that.
Cefiderocol has been approved by the US Food and Drug Administration and by the European Medicines Agency, and is included on the WHO Model List of Essential Medicines. Until now, this antibiotic has been available in only a few high-income countries, and it has not been available at all in low- and middle-income countries (LMICs). On the basis of a first-of-its-kind license agreement with Shionogi, a collaboration agreement with Shionogi and CHAI, and a manufacturing sublicense agreement with Orchid Pharma, GARDP and partners aim to forge new pathways and build sustainable networks so that cefiderocol—and, in the future, other antibiotics like it—can reach people in need.
Under the agreement with Shionogi, GARDP will be able to manufacture and commercialize cefiderocol through sub-licensees in almost 70% of countries worldwide (135 countries), most of which tend to have delayed access (if any) to novel antibiotics. The license territory includes all low-income countries, most lower middle- and upper middle-income countries, and select high-income countries. The manufacturing sublicense agreement with Orchid Pharma Ltd (Orchid) has important access, environmental, & stewardship provisions, to help keep the product affordable for patients and health systems in low-resource settings.






Implementer networks and other activities
A number of barriers hinder new antibiotics from being available for the right patient at the right time, especially in low- and middle-income countries. To overcome these barriers, GARDP is facilitating the development of implementer networks in Kenya and South Africa, as well as in India. Similarly, it is engaging stakeholders in the Latin American and the Caribbean region, with an initial focus on Brazil.
These implementer networks bring together members of national Ministries of Health, academic centres and major public and private healthcare providers to help identify which new antibiotic treatments are most important for their region. They may act as early adopters of these treatments and share data and best practices to introduce these treatments appropriately across the country or region. Although GARDP currently has a coordinating role of these networks, the goal is that these will become self-managed and self-funded in the future.
Alongside these networks, GARDP carries out a number of market shaping and policy-related activities to foster antibiotic access, including generating data to offer insight on product demand and appropriate use, and recommending policies that enable reliable and affordable antibiotic supply.



SECURE: Expanding Access to Antibiotics
SECURE is a collaborative initiative between GARDP and WHO aimed at supporting countries in their efforts to expand access to essential antibiotics. Its aim is to catalyze new models to accelerate access to a portfolio of essential antibiotics in LMICs. This includes generic antibiotics that are in short supply or not widely available, as well as newly approved “Reserve” antibiotics for resistant bacterial infections.
By improving efficiencies in forecasting, procurement and supply, SECURE aims to improve decision-making, reduce shortages and identify priorities for local manufacturing. SECURE has also developed a forecasting and economic model to illustrate the benefits of pooling demand, stockpiling, supplier guarantees and country subsidies, all of which can help to incentivize suppliers.
Also, improvements in supply security, demand predictability, availability and affordability of these antibiotics will help to expand access in a sustainable, equitable and appropriate way.
SECURE is the first dedicated initiative to expand access to a portfolio of essential antibiotics in LMICs. This goes beyond access to single products.

Our vision for access
Key milestones
Key milestones
- 2017-2022: SIGNED collaboration and license agreements with: Entasis Therapeutics, an affiliate of Innoviva Specialty Therapeutics, for zoliflodacin (2017); Venatorx Pharmaceuticals for cefepime-taniborbactam (2020); Shionogi & Co., Ltd, and CHAI for cefiderocol (2022)
- 2021: PARTNERED with InfectoPharm, which donated supplies to support the development of new treatment for neonatal sepsis
- 2021: SIGNED memorandum of understanding with the Thailand Ministry of Public Health to work toward ensuring access to the antibiotic zoliflodacin
- 2023: SIGNED manufacturing sublicense agreement with Orchid Pharma
- 2024: SIGNED collaboration agreement with the Global Drug Facility to pool procurement of antibiotics in GARDP portfolio
- 2024: SIGNED collaboration agreement with Pan American Health Organization (PAHO) to improve regional antibiotic R&D, manufacturing and access in the context of the emergence and spread of antibiotic-resistant infections in Latin America and the Caribbean
Learn more
Learn more
- Meeting Report: Strengthening Antibiotic Access in India
- Access fact sheet: Challenges to sustainable access for AMR treatments
- Poster on antibiotic implementor networks in South Africa and Kenya (PREACTs)
- Assessment of Antimicrobial Resistance Diagnostic Capacity and Antibiotic Use in 10 Counties in Kenya
- Access Strategy and Priorities (Updated Nov. 2023)
- Cefiderocol access project: Overview of license agreement with Shionogi (see also full license agreement) (2022)